Eucharitidae
Primary Research Projects - John Heraty
- Revisionary systematics of the genus Orasema (John Heraty, Jason Mottern, Judith Herreid, Austin Baker)
- Molecular systematics and biogeography of the poneromorph-parasitic Eucharitidae (Elizabeth Murray)
- World catalog of the species of Eucharitidae, 2019 (John Heraty) [eucharitid.catalog.pdf]
- Literature for descriptive taxonomy of Eucharitidae [references]
Universal Chalcidoidea Database family description: Eucharitidae
Recent Publications
- Heraty, J.M., Murray, E. 2013. The life history of Pseudometagea schwarzii, with a discussion of the evolution of endoparasitism and koinobiosis in a specialized group of chalcid wasps. Journal of Hymenoptera Research 35: 1–15.
- Heraty, J.M. Burks, R.A., Cruaud, A., Gibson, G.A.P., et al. (27 authors). 2013. A Phylogenetic analysis of the megadiverse Chalcidoidea (Hymenoptera). Cladistics, 29: 466–542. doi/10.1111/cla.12006
- Murray, E., Carmichael, A.E. and Heraty, J.M. 2013. Ancient host shifts followed by host conservatism in a group of ant parasitoids. Proceedings of the Royal Society, B. 280: 0130495. (9 pages)
- Torréns, J. and Heraty, J.M. 2013. A new genus of Eucharitidae (Hymenoptera: Chalcidoidea), with notes on life history and immature stages. Zootaxa 3630: 347-358.
- Torréns, J. and Heraty, J.M. 2012. Description of the species of Dicoelothorax Ashmead (Chalcidoidea, Eucharitidae) and biology of D. platycerus Ashmead. Zookeys 165: 33-46.
- Carey, B., Visscher, K. and Heraty, J. 2012. Extrafloral nectaries for gaining access to an ant host by the parasitoid Orasema simulatrix (Hymenoptera: Eucharitidae). Journal of Hymenoptera Research 27: 47–65.
- Varone, L., Heraty, J.M., Calcaterra, L.A. 2010. Distribution, abundance and persistence of species of Orasema (Hym: Eucharitidae) parasitic on fire ants in South America. Biological Control 55: 72-78.
- Heraty, J.M. and D.C. Darling. 2009. Fossil Eucharitidae and Perilampidae (Hymenoptera: Chalcidoidea) from Baltic Amber. Zootaxa 2306: 1-16.
- Heraty, J.M. (2002) A revision of the genera of Eucharitidae (Hymenoptera: Chalcidoidea) of the World. Memoirs of the American Entomological Society, 68, 1–359.
- Heraty, J.M. (2000) Phylogenetic relationships of Oraseminae. Annals of the Entomological Society of America, 93, 374–390.
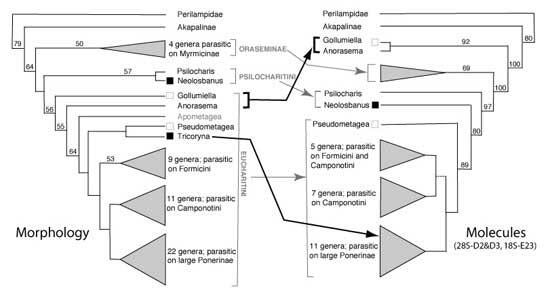
The Eucharitidae are a specialized group of ant parasites. The genera were revised by Heraty (2002). Hypotheses of relationships are being developed using morphological characters of the adult and immature stages, and through analysis of molecular data from ribosomal and mitochondrial. Results from an analysis of 75 characters scored from adults indicate a strong correlation between the relationships of Eucharitidae and their ant hosts: parasitism of Myrmicinae appears correlated with use of a thrips intermediate host to gain access to the ant brood, parasitism of Ponerinae is correlated with independent larvae, and parasitism of Formicinae is associated with the attraction of ants to the egg mass. The molecular analysis by Murray et al. (2013)
- 56 genera
- 466 species
Distribution: worldwide, mostly in tropics; very few genera shared between the Old and New World.
Classification: Eucharitidae consist of three monophyletic subfamilies, Gollumiellinae, Oraseminae and Eucharitinae (Munro et al. 2011; Heraty et al. submitted). The Australian subfamily, Akapalinae, may be the sister group of the remaining subfamilies, although it position is variable in recent analyses using ribosomal (18S E17-35, 28S D2-D5) and cytochrome oxidase (COI&II) data. Gollumiellinae is an odd subfamily that is defined almost solely by molecular features. Females eucharitids deposit their eggs in or on plant tissue and are parasites of ant pupae through the larval stage of the host (Clausen 1940ab, 1941; Heraty 1994a, 2002). First-instar larvae and adults possess several character states that justify the monophyly of Eucharitinae + Oraseminae, and provide reliable evidence for determining phylogenetic relationships with respect to Perilampidae (Heraty & Darling 1984; Heraty 1994a) and Philomidinae (Darling 1992). Oraseminae are monophyletic and form the plesiomorphic sister group of the Eucharitinae (Heraty 1994a). The Oraseminae is comprised of four genera, three of which are found in the Old World tropics and Orasema, which is circumtropical.
Eucharitinae is comprised of two tribes: Psilocharitini, which includes two genera in the Old World (Heraty 1994a); and Eucharitini, which is a morphologically diverse group of 36 described genera worldwide (49 recognized; Heraty, unpublished). Psilocharitini may prove not to be monophyletic but rather a paraphylstic sister groups of the Eucharitini. Monophyly of Eucharitini (Eucharitinae s.s.) is supported by anterior fusion of the prepectus and the pronotum, with complete enclosure of the mesothoracic spiracle internally; loss of the antennal ring segment; and the first phragma forming a strong anterior ridge internally above the dorsal margin of the pronotum (Heraty 1985, 1989; Boucek 1988). The monophyly of Eucharitinae is supported by additional characters of the first-instar larva, which include fusion of tergites 1&2, the absence of cranial setae and the presence of a labial plate (Heraty & Darling 1984, 1994a). Interestingly, Gollumiellinae have a fused pronotum and prepectus, a fused first and second tergite in the first-instar larva, and either have an anellus or a partially to completely fused first and second flagellomere (Heraty et al. 2004).
The molecular phylogeny strongly supports a sister group relationship between Gollumiella + Anorasema and Oraseminae + Eucharitinae (Heraty 2003, Munro et al. 2011, Heraty et al. submitted). Gollumiella longipetiolata Hedqvist are parasitoids of Paratrechina (Formicidae: Formicinae). Adults deposit their eggs vertically on the stalks of leaf fronds of Cyathea latebrosa (Cyatheaceae) in a manner similar to that described by Clausen (1940) for G. antennata Gahan. The shift of Tricoryna to the clade of large ponerine parasites (Ectatommatini and Odontomachini) and sister group of the genus Austeucharis makes more sense than the morphological hypothesis. Studies are underway to sequence a large number of genera across Eucharitidae and several outgroups to derive a phylogeny to compare against the morphological phylogeny presented in Heraty (2002).
Biology: A preliminary analysis of the phylogeny and a reclassification of the Oraseminae and Eucharitinae indicate a strong correspondence between related groups of parasites and their ant hosts (Fig. 1, Table 1). Within ant subfamilies host relationships are often confined to related genera such as Clade 1 (Fig. 1), which are almost all parasites of Odontomachus (Ponerinae). The ant genus Pheidole (Myrmicinae) is proposed as the ancestral host for species of Orasema and possibly Oraseminae (Table 1; Heraty 1994a,b). Eucharitinae are indicated as parasites largely of poneromorph ants (Ectatommatinae, Ponerinae) and Formicinae, with one Australian genus parasitic on the bull-dog ant (Myrmeciinae) (Heraty 1994a). Eucharitids parasitic on Formicinae are currently hypothesized as belonging to one or more derived lineages of Eucharitinae. Species of Orasema do attack some of the economically important species of ants such as the imported fire ant, Solenopsis invicta, in southern South America, and the little red fire ant, Wasmannia auropunctata, in the Caribbean (Heraty 1994).
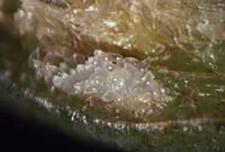
Eggs of eucharitids are deposited into plant tissue. Gollumiella have a unique behavior involving hooking the anchor-like basal tip of the egg into plant tissue, and then depositing a ropey secretion to the apex of the egg, which stands erect in the host plant. Presumably, the secretion acts as an attractant for its host formicine, Paratrechina (Heraty et al. 2004). Oraseminae and Psilocharitini deposit their eggs into punctures made by the ovipositor, Eucharitini all deposit their eggs into preformed cavities (plant buds or inflorescences), onto the undersurface of leaves, or into the outer skin of fruits. The habit of oviposition into the leaf surface has resulted in some species of Orasema being categorized as pests (O. costaricensis on bananas in Costa Rica; Orasema species in Argentina on olives; O. assectator on leaves of tea), although this is a rare phenomenon and probably the result of an imbalance in the system, possibly by DDT in the case of the banana infestations during the early 60's.
| Eggs of Obeza floridanain fruit | Egg punctures of Orasema costaricensis |
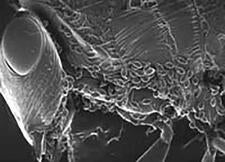
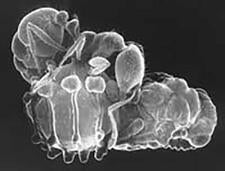
The first-instar larvae are minute, less than 0.13 mm, strongly sclerotized, active, and are responsible for gaining access to the ant host. Access to the host ant colony is by various means (see Clausen 1940abc). Generally, parasitoids of ponerine ants have very active larvae that will attach to almost anything that moves, presumably attaching to foraging ants in the field. This behavior can lead to specimens attaching to females of the same species that are ovipositing later on the same plant. Parasitoids of Campanotini, such as Obeza, either oviposit into fruit, or deposit their eggs as large masses that may resemble fruits, and appear to access the ants by recruitment to the egg mass itself (Heraty & Barber 1990). The myrmicine parasites, in the subfamily Oraseminae, use various behaviours, but in most cases an intermediate thrips or auchenorrhycan host is involved, with planidia usually attaching lightly to the adbomen of the thrips or leafhopper. Their primary hosts, mostly Pheidole and Solenopsis, are predators of small thrips and may be carrying the food source, with planidia, directly to the ant larval host. Differing from other members of the genus, Orasema simulatrix deposits eggs around extrafloral nectaries of Chilopsis and the emerging planidia then migrate into the liquid-filled nectaries, which are tending by their host Pheidole desertorum.
| Planidia on Kapala floridana | Planidium (first-instar) on tip of insect pin |
Larval development takes place in the ant nest. Eucharitine planidia attach externally to the host larva, whereas Gollumiellinae and Oraseminae burrow into the host thorax just behind the head and do limited feeding (slight expansion of tergites). Further development does not take place until the host pupates. In all cases, the first instar larvae migrate to the ventral region of the host pupa where they feed until most or all of the host is consumed. Only one host is consumed and only very rarely do more than one eucharitid develop on a host. Adults emerge within the nest and may remain for a period of time (unknown how long) within the nest. Adults, and immature stages where exposed, are generally well treated within the nest by the ants, which will feed them, groom them or carry them away to protect them if the colony is under attack. Adults must leave the nest to mate and lay eggs.
| Solenopsis invicta carrying pupa of Orasema xanthopus |
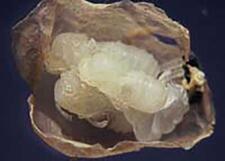
Pupae of Pseudochalcura gibbosa in coccon of Camponotus herculeanus |
____________________________________________________________________________________
If you collect any eucharitids or ants with suspected eucharitid parasites, please contact john.heraty [at] ucr.edu